Patent
Patent Related to Organic Chemical Synthesis
Synthesis of Polyphenyl Compounds Without Using Organic Solvents
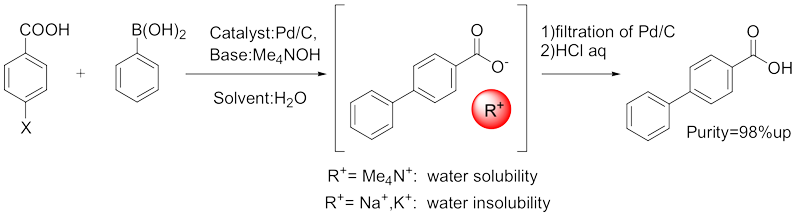 |
|
Patent No. 5222632
Polyphenyl carboxylic acids (sulfonic acids) are widely used as important structures in pharmaceuticals and functional organic compounds. These compounds can be manufactured using the Suzuki cross-coupling reaction, which won the Nobel Prize in Chemistry. While this reaction is highly useful and widely applied, the manufacturing methods for polyphenyl carboxylic acids have the following issues: ①A large amount of organic solvents is required during manufacturing, leading to cost issues, residual solvent issues, and problems for humans and the environment. ②There is a risk of fire due to static electricity from organic solvents during the purification process. ③The use and removal of expensive palladium metal, which belongs to rare metals, is complicated. ④Polyphenyl carboxylic acids and their Na salts are insoluble in water, making separation from heterogeneous catalysts difficult. To address these challenges, the R&D Center developed a manufacturing process that uses inexpensive and harmless water as a solvent instead of organic solvents, along with recoverable metal catalysts, achieving a safe and easy manufacturing process. This manufacturing technology has been successfully put into practical use. The manufacturing process developed by the R&D Center combines easily obtainable Pd/C and tetraalkylammonium hydroxide as a base, enabling the Suzuki cross-coupling reaction in water without using any organic solvents, and allowing easy synthesis of polyphenyl carboxylic acids. This method is very simple to operate, produces minimal waste, and is a clean process. The target product obtained by this method is of high purity and does not require purification. Additionally, the Pd/C removed by filtration does not pose a fire hazard as the reaction solvent is water, eliminating the need for special filtration equipment. Furthermore, the recovered Pd/C can be reused in the same reaction without any purification treatment. |
Method for producing Sepiapterin and Tetrahydrolactoylpterin
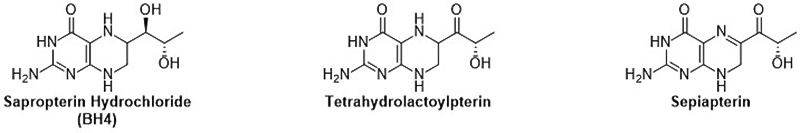 |
|
Patent No.6084967 International Publication Number WO2013/168693 Patented in 27 countries including Japan and the United States. Sepiapterin and Tetrahydrolactoylpterin are natural substances with the same pterin skeleton as Sapropterin dihydrochloride (BH4), which has various functions in the body. Both compounds are intermediates in the BH4 biosynthesis pathway. These compounds are converted to BH4 in the body and function as BH4. Recent studies have shown that Sepiapterin can pass through the blood-brain barrier more easily than BH4 and may improve brain dysfunctions that BH4 could not address*1. BH4 is known to be involved in various diseases, and clinical research has been conducted on many diseases, but only PKU treatment has been commercialized. These compounds are expected to be promising new drug candidates effective for various diseases as alternatives to BH4. However, until now, synthesis methods for these compounds have not been developed, and a stable supply method needed to be established for their practical use as pharmaceuticals. 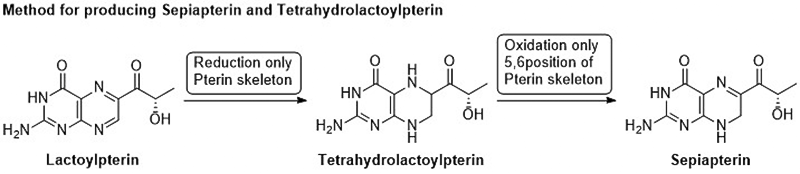 The manufacturing method developed by the R&D Center uses Lactoylpterin, which can be synthesized by general methods, as a raw material. Without using protecting groups, Tetrahydrolactoylpterin is synthesized in one step by a reduction reaction, followed by Sepiapterin synthesis in one step by an oxidation reaction. These compounds have a carbonyl group in the side chain, making synthesis difficult by conventional reduction methods. The newly developed method can selectively reduce only the pterin ring without retaining the carbonyl group, successfully synthesizing natural Tetrahydrolactoylpterin for the first time in the world. Additionally, Tetrahydrolactoylpterin is positioned as an important intermediate of Sepiapterin, and a method to selectively oxidize only the 5,6 positions of the pterin ring was developed, achieving a practical method for producing Sepiapterin. *1: WO2011/132435 Drug and Food for Prevention and Improvement of Brain Dysfunction |
Method for producing Hydrazone derivatives
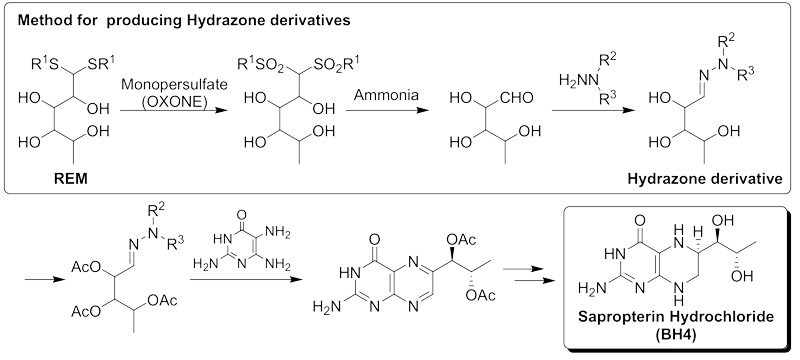 |
|
Patent No.5042843 International Publication No.WO2006/118322 Patented in 28 countries including Japan and the United States. Sapropterin dihydrochloride (BH4) is present in the body and is involved in the synthesis of neurotransmitters such as serotonin, dopamine, noradrenaline, and adrenaline, as well as the synthesis of nitric oxide (NO), a signaling molecule. A deficiency of BH4 in the body can lead to various disorders. Phenylketonuria (PKU) is one such disorder, which can cause severe developmental issues in the brain, necessitating early detection. In Japan, all newborns are screened for PKU. BH4 is an important compound as the only treatment for PKU. However, BH4 is extremely expensive, thus efficient and economical manufacturing methods were sought. Hydrazone derivatives are crucial intermediates in the production of BH4. Although many methods for synthesizing Hydrazone derivatives have been reported, they are unsuitable for large-scale production due to issues such as lack of reproducibility, low yields, the use of dangerous peroxides, and the need for concentration and column purification. Ensuring a stable supply of Hydrazone derivatives was the biggest challenge for providing BH4 at a low cost. First, the cause of the yield reduction in Hydrazone derivatives was identified as the oxidation process of REM, and a new oxidation method was developed at the R&D Center. Subsequently, a continuous and large-scale synthesis process for Hydrazone derivatives was developed, and the manufacturing technology was successfully put into practical use. The process developed at the R&D Center uses chemically stable OXONE® as an oxidizing agent, avoiding dangerous oxidation reactions and enabling quantitative oxidation reactions with minimal side reactions. Additionally, the method from REM to Hydrazone derivatives can be carried out continuously in water, allowing quantitative production of Hydrazone derivatives without purification or concentration operations. The manufacturing period is only three days, making it significantly more efficient compared to conventional methods. Furthermore, the continuous reaction in water results in a process with very low environmental impact. |
Development of PGC1α Expression Promoters and Fat Reduction Promoters
 |
|
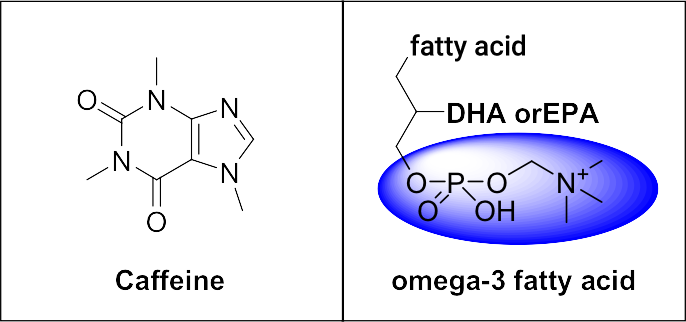
Patent No.6420645(PGC1α Expression Promoter) Patent No.6426991(Body Fat Reduction Promoter) Globally, the number of obese individuals is increasing, and obesity has various adverse effects such as increased burden on the heart, increased stress on joints like knees and hips, and causing lifestyle-related diseases. Obesity is a condition where excess neutral fat accumulates in the body. To improve obesity, it is necessary to suppress the absorption of neutral fat, decompose the accumulated neutral fat, and consume(burn) the free fatty acids generated from the decomposition as energy. If the free fatty acids generated from the decomposition of neutral fat are not consumed, they are re-synthesized into neutral fat and accumulate again in fat cells. If mitochondria, which consume free fatty acids and carbohydrates, can be increased and activated, it may be possible to improve obesity by enhancing basal metabolism without the need for intense exercise. The protein that influences the increase and activation of mitochondria is PGC-1α (Peroxisome proliferator-activated receptor γ coactivator-1α), which controls gene transcription. Through extensive research, we found that the combination of caffeine and phospholipids containing ω3 unsaturated fatty acids shows a synergistically superior effect in promoting PGC-1α expression in visceral fat cells and muscle cells compared to when each is used alone. This invention also has the effect of promoting the decomposition of neutral fat and inhibiting the differentiation of precursor cells into fat cells. In other words, this product is expected to promote the consumption of free fatty acids through the increase and activation of mitochondria from the decomposition of neutral fat even at rest. Therefore, it is considered to be a useful substance for improving basal metabolism, with potential benefits for various lifestyle-related diseases and anti-aging effects. |







